number of electrons of nitrogen|Nitrogen (N) : Manila Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to .
A Relic-tier sword available only with the Hearts of Stone DLC, Iris is solidly at the top of most Witcher 3 players' best steel swords tier lists. As well as just being a really, really powerful weapon, Iris has .
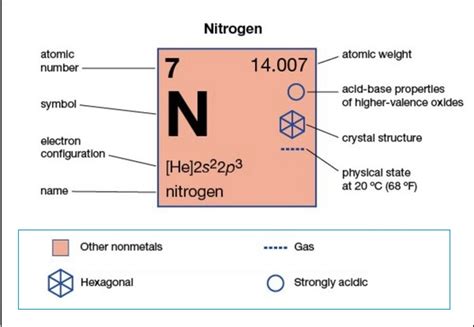
number of electrons of nitrogen,Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe temperature at which the .The atomic number of each element increases by one, reading from left to .The atomic number of each element increases by one, reading from left to . The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number .

Nitrogen is the 7th element in the periodic table and has a symbol of N and atomic number of 7. It has an atomic weight of 14.007 and a mass number of 14. Nitrogen has seven .
Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to .
z. It, therefore, has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), .
Therefore, the number of electrons in neutral atom of Nitrogen is 7. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Since the .The total number of electrons in nitrogen is seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in nitrogen in specific rules in different orbits and orbitals is .Electrons: 7: Protons: 7: Neutrons in most abundant isotope: 7: Electron shells: 2,5 : Electron configuration: 1s 2 2s 2 2p 3: Density @ 20 o C: . Over time, each carbon-12 nucleus can take part in a very large number .Nitrogen is a chemical element of the periodic table with chemical symbol N and atomic number 7 with an atomic weight of 14.0064 u and is classed as a nonmetal. . Number .The number of unpaired electrons in the last orbit of an element is the valency of that element. As we know, the correct electron configuration of nitrogen in ground state will be 1s 2 2s 2 2p x1 2p y1 2p z1. Here, the nitrogen atom has three unpaired electrons. Therefore, the valency of nitrogen is 3. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Nitrogen. From the Periodic Ta.The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons. In general, atoms are most stable, least reactive, when their outermost electron shell .
The simplest compound of nitrogen is molecular nitrogen, N2 N 2. The two nitrogen atoms are bonded together by a triple bond, consisting of a σ σ and two π π bonds. Molecular nitrogen, N2 N 2 [8] A common nitrogen-containing molecule is ammonia ( NH3 N H 3 ), which is analogous to methane ( CH3 C H 3 ). In ammonia the nitrogen atom is .
The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. . Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals.
4th shell can hold 32 electrons. Now the atomic number of Nitrogen (N) is 7. Hence nitrogen element has the electrons arrangement 2, 5. This electron arrangement indicates that the outermost orbit of Nitrogen (N) has 5 electrons. Hence, it lies in group 15.Nitrogen atomic number and atomic weight. We know that the atomic number of nitrogen is 7 and the atomic mass number is 14. Neutron (n) = 14 – 7 = 7. Therefore, the number of neutrons in nitrogen is 7. Based on the atomic number of the element, the mass number, and the number of neutrons, three things can be considered.
Nitrogen: Symbol: N: Atomic Number: 7: Atomic Mass: . Number of Neutrons: 7: Number of Electrons: 7: Melting Point-209.9° C: Boiling Point-195.8° C: Density: 1.2506 grams per cubic centimeter: Normal Phase: Gas: Family: . Nitrogen gas is created through the distillation of liquid or gaseous air.Nitrogen (N) Atomic Number of Nitrogen. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons . The valence electrons of nitrogen in its compounds are all sp³ hybridized orbitals. The formal charge on N is usually -1 for an anion, 0 for a neutral compound, and +1 in cations. A nitrogen atom with a formal charge of -3 would correspond to a nitride ion, .
Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. . Nitrogen shares its electrons with the chlorine atoms, so .
Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Thus, a nitrogen atom will form an anion with three more electrons than protons .(b) In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compounds ? (c) The electronic configuration of nitrogen is (2, 5). How many electrons in the outer shell of a nitrogen atom are not involved in the formation of a nitrogen molecule ? The electron configuration of Nitrogen “Electron configuration is the distribution of electrons of an atom or molecule in atomic or molecule orbitals” Nitrogen has an atomic number of 7 and it contains a total number of 7 electrons. From the Bohr model of Nitrogen, we know, that 2 electrons are in the K-shell and 5 electrons are in .

Nitrogen, with an atomic number of 7, has an electron configuration of 1s^2 2s^2 2p^3. This means that nitrogen has a total of seven electrons distributed in different energy levels or orbitals. Starting from the innermost shell, nitrogen has two electrons in the 1s orbital, two electrons in the 2s orbital, and three electrons in the 2p orbital. You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are . the real behavior of electrons is less simple. Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. . Nitrogen-3, .There are always the same maximum number of electrons in orbitals with the same symbol, even if they are from different shells, s always maxes out at 2, p at 6, d at 10, f at 14 etc., each increasing by 4. Following the Lowest Energy Principle, electrons will always go into the lower energy atomic orbitals first.Nitrogen Energy Levels. With an electron configuration of 1s 2 2s 2 2p 3, the element nitrogen has three electrons outside closed shells. The three spins can give a resultant of spin 3/2 (quartet states) or 1/2 (doublet states). In the diagram above, it is presumed that two of the electrons remain in their lowest states, and the lower case .
number of electrons of nitrogen|Nitrogen (N)
PH0 · Protons, Neutrons, Electrons for Nitrogen (N, N3−)
PH1 · Nitrogen Element Facts
PH2 · Nitrogen (N)
PH3 · Nitrogen
PH4 · Electron Configuration for Nitrogen (N and N3
PH5 · 8.9.2: Chemistry of Nitrogen (Z=7)